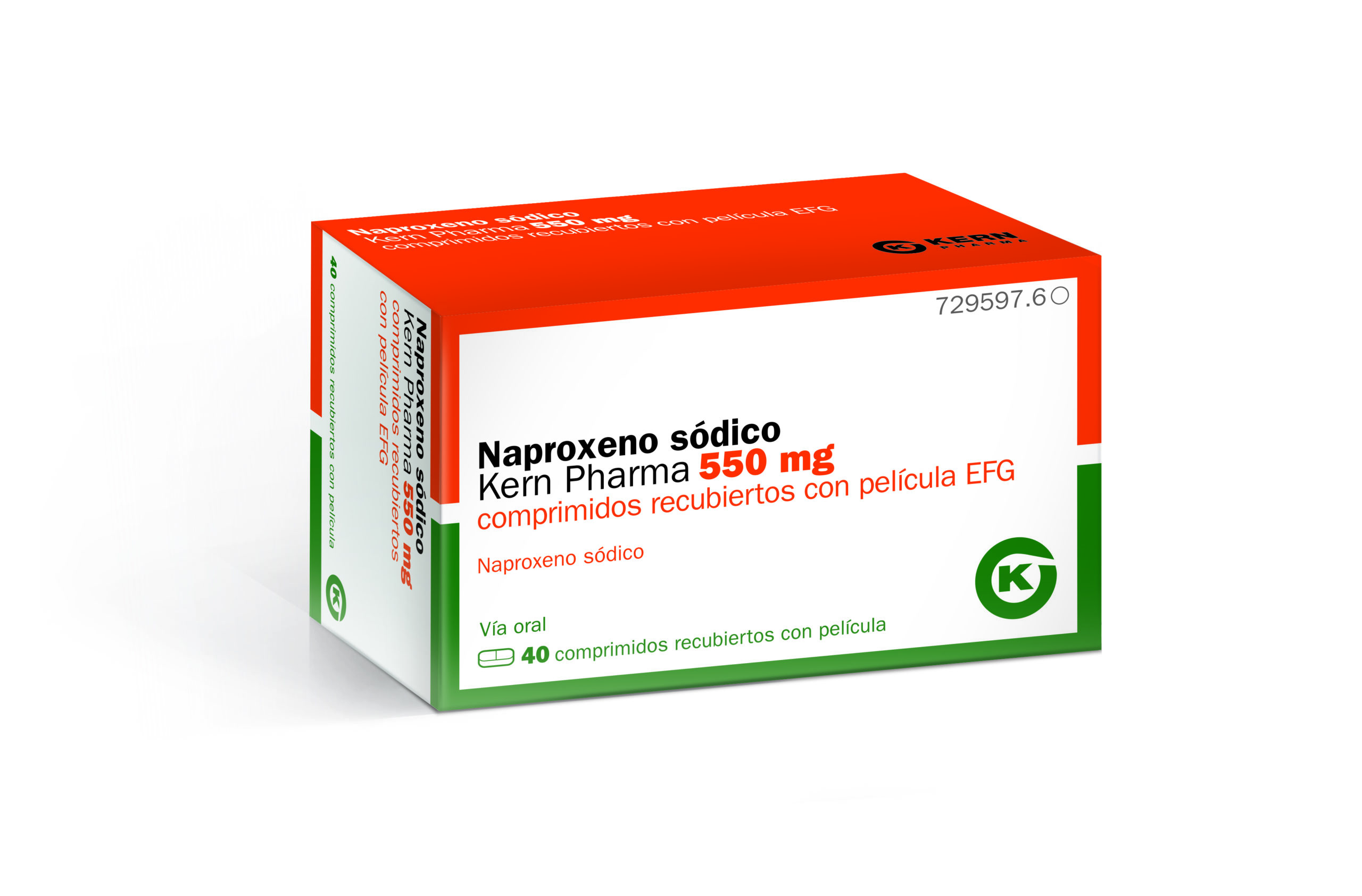
PHARMACEUTICAL DOSE FORMS
- FILM-COATED COMPRESSED
ROUTES OF ADMINISTRATION
- ORALLY
STRENGTH
- 550 MG
ACTIVE INGREDIENTS
- SODIUM NAPROXEN
EXCIPIENTS
- SODIUM LAURYLSULFATE
- CARBOXIMETHYLMIDONE SODIUM TYPE A
CHARACTERISTICS
- MEDICINE SUBJECT TO MEDICAL PRESCRIPTION
- MEDICAL PRESCRIPTION
ATCCODES
- M01A – NON-STEROID ANTI-INFLAMMATORY AND ANTIRREUMATIC PRODUCTS
- M01AE – PROPIONIC ACID DERIVATIVES
- M01AE02 – NAPROXEN